More news
- Focus on the global coatings market: Global coatings market outlook
- View from the UK: Navigating chemical policy and sustainability
- Focus on adhesives: Unveiling unbreakable bonds – Testing redefines physical strengt...
- Focus on adhesives: Henkel and Covestro collaborate for sustainability of engineered wood ...
- Advances in construction chemical technology: What’s new in 2024?

In this article, Artur Palasz, Spektrochem, dives into the topic of water: An often overlooked but incredibly vital raw ingredient in the coatings industry
Water in waterborne paints, also called water-based as the name suggests, is treated almost as the main ingredient. Born from water, these paints are gradually replacing solventborne paints through research and are now comparable to industrial coatings, constituting a more friendly alternative both in terms of the environment, VOC content, as well as ease of use. There is also no need to use organic solvents for washing equipment after painting. There are also some limitations of waterborne paints, such as the impossibility of application at low temperatures, e.g. below 32°F / 0°C, which requires appropriate application and drying conditions for waterborne paint coatings.
However, this does not change the fact that waterborne paints constitute approximately 60% of the global coatings market and their largest share are architectural paints and paints from the deco sector, including wood-coatings.
Water in waterborne paints is a carrier, a solvent and a diluent at the same time. This is where fillers and pigments are dispersed in the production of paints, where the emulsion polymerisation process takes place when synthesising latex polymer dispersions, and where all intermediate raw materials are dissolved or suspended in water, such as cellulose thickeners to form a gel, or smectites for creating a suspension and further using them as rheological additives. Water is also used to adjust viscosity during painting, as well as to wash tools after painting.
Water is also used to test coatings in laboratories: Tests include the resistance of coatings to water; the use of water in accelerated weathering equipment where water is heated and condensation is produced on the coatings; or in the case of simulating rain during water spray. Water is also used in coating tests to prepare solutions of aggressive substances to test the resistance of coatings, e.g. to various concentrations of solutions of acids, alkalis, salts, etc.
Water is used everywhere in these applications. But what kind of water? Demineralised, tap water, hard or softened… and if demineralised, what kind? Because there is not only one demineralised water. In this technical article, I invite you on a journey through the most valuable resource in the world: Water, which in the case of paints is often overlooked and treated too generally. This article discusses an extremely important component of paints, in the case of which it is not possible to clearly answer which type of water is best for the production of paints, but dives deep into the issue to explain in which cases a specific type of water should be used to produce waterborne paints.
One water, many waters
Water is a chemical compound with the formula H2O, existing in the liquid state under standard conditions. Everyone can say that they have come into contact with sea (salt) water, river water, tap water, demineralised water, potable water, purified water in a bottle, etc. So, as you can see, there is no one type of water. To be precise, chemically it is still H2O, but there is something in each of these waters that distinguishes them – and it is mainly about what is dissolved in them. Due to the fact that water is a good solvent for polar substances, various compounds, such as salts, dissolve in it, but microorganisms, such as bacteria, and insoluble but suspended solid particles, such as minerals, can also be found in water. This means that we are dealing with various names for water and their definition, e.g. suitability for consumption or specific industrial uses.
Depending on habits and awareness of water quality, there are several models for using water to produce waterborne paints. Here are a few of them:
- Using tap water (not aware of what is in it)
- Using tap water and “softening” it with some additives (the cognitive errors associated with this are explained later in the article)
- Using demineralised water (more or less controlled in terms of parameters)
- Using recycled washing water from previous production, often also demineralised
Let’s start our discussion about water with the classification of different types of water used to produce paints. I will focus on discussing two types of water: demineralised water and tap water, as I do not assume that anyone uses directly from the river to produce paints. Water from recycling or purification processes, e.g. by adding coagulants that bind paint particles after washing e.g. tanks or production dissolvers, can also be used, however, the water purification process must lead to obtaining water with a specific degree of conductivity and microbiological purity. Therefore, regardless of whether the water purification process involves distillation or adding coagulants and then, for example, demineralisation, it should lead to obtaining water with parameters as discussed in the parameters for demineralised water or tap water.
Demineralised water
Demineralised water is water from which the salts and other minerals dissolved in it have been removed. Removal of salts and minerals takes place in the process of distillation, ion exchange (DI), continuous electrodeionisation (EDI), reverse osmosis (RO) or a combination thereof. Demineralised water is classified most extensively by the ASTM D1193 standard (see Table 1), which, depending on the type and grade differing in the water production process, distinguishes parameters such as electrical conductivity (µS/cm), resistivity (MW · cm), pH, total organic carbon (TOC), sodium content, chloride ion, total silica and microbiological purity as the amount of heterotrophic battery count and endotoxin.
| Table 1. ASTM D1193 demineralised water classification | |||||||||||
| Type | Grade | Process | µS/cm
(max.) |
MW·cm
(min.) |
pH | TOC
µg/L (max.) |
Sodium
µg/L (max.) |
Chloride
µg/L (max.) |
Total silica
µg/L (max.) |
HBC
cfu/mL (max.) |
Endotoxin
EU/mL (max.) |
| I | – | Purify to 20 µS/cm by distillation or eqv., followed by mixed bed DI, 0.2 µm filtration | 0.0555 | 18 | – | 50 | 1 | 1 | 3 | – | – |
| A | 10/1000 | 0.03 | |||||||||
| B | 10/100 | 0.25 | |||||||||
| C | 100/10 | – | |||||||||
| II | Distillation | 1.0 | 1.0 | – | 50 | 5 | 5 | 3 | – | – | |
| A | 10/1000 | 0.03 | |||||||||
| B | 10/100 | 0.25 | |||||||||
| C | 100/10 | – | |||||||||
| III | – | Distillation, DI, EDI, RO or a combination thereof followed by 0.45 µm filtration | 0.25 | 4.0 | – | 200 | 10 | 10 | 500 | – | – |
| A | 10/1000 | 0.03 | |||||||||
| B | 10/100 | 0.25 | |||||||||
| C | 100/10 | – | |||||||||
| IV | – | Distillation, DI, EDI, RO or a combination thereof | 5.0 | 0.2 | 5.0 – 8.0 | – | 50 | 50 | – | – | – |
| A | 10/1000 | 0.03 | |||||||||
| B | 10/100 | 0.25 | |||||||||
| C | 100/10 | – | |||||||||
In addition to all other parameters, conductivity is the key associated with demineralised water and it may be noticed that each type of demineralised water classified according to ASTM D1193 has a specific maximum conductivity limit and therefore, requirements for total organic carbon, dissolved chloride and sodium ions and silica content. Individual Grades are differentiated mainly in terms of microbiological purity. Demineralised water classified in ASTM D1193 is used in laboratories primarily as reagent water, however, depending on the type of water, this standard is also helpful in classifying demineralised water for industrial uses, including potential use in paints.
Another standard is ISO 23321 which specifies requirements for demineralised water used as solvent for paints and varnishes industrial applications, e.g. production of electro-deposition coating materials, water-based coating materials, water-based resins and plastics dispersions. Standards ISO 23321 defining demineralised water as water of which the mineral matter or salts have been removed by deionisation and determining its specification as shown in Table 2. As you can see, standard has fewer requirements and a much higher limit for conductivity.
| Table 2. Requirements for demineraliaed water for paints according to ISO 23321 | |
| Property | Requirement |
| Appearance | clear, without foreign matter |
| Electrical conductivity at 25 °C | max. 20 µS/cm |
| pH-value at 23 °C | 5 to 8 |
| Chloride content | max. 3 mg/l |
| Evaporation residue | max. 5 mg/kg |
| Colony content | without findings CFU |
Tap water
Tap water is not always drinking water. In this respect, the classification of parameters that water must meet in terms of suitability for drinking is determined by local requirements set by authorised sanitary institutions in individual countries. In the case of tap water, of course, microbiological parameters, but what is interesting from the point of view of industrial applications for paint production is water hardness. It is often believed that water softening is necessary for paint production. Local requirements do not define the conductivity of tap water (usually), but indicate the hardness level, because depending on the water intake and the method of its treatment, water of varying hardness is obtained.
Water hardness is a characteristic of water that is a function of the concentration of calcium (Ca2+) and magnesium (Mg2+) cations, and optionally iron (Fe2+) and manganese (Mn2+). Water hardness affects its surface tension and conductivity. The greater the surface tension of water, the more difficult it is to wet any surface. Depending on the region of the world, water hardness is expressed in units:
- Grains per US gallon (gpg) – 1 gpg = 1 grain of CaCO3 in US gallon (3.79 L)
- French degrees (°f or °fH) – 1 °f = 10.00 mg CaCO3 in 1 liter of water
- English or Clark degrees (°e) – 1 °e = 1 grain (64.8 mg) CaCO3 in an English gallon (4.55 L)
- German degrees (°n or °d or °dH or dGH) – 1 °n = 10.00 mg CaO in 1 liter of water = 17.86 mg CaCO3 in 1 liter of water (in German-language literature marked as °dH (Grad deutscher Härte),
- Millivals per liter (mval/l) – 1 mval = 1 milligram equivalent (0.5 mmol) of Ca2+ ions and 1 mval = 50 mg CaCO3 in 1 liter of water
Water hardness is determined using laboratory methods using classical analysis (titration), however, due to the time-consuming analysis process, it is replaced by the dependence of conductivity on water hardness and is determined using electrical conductivity measurements, especially using a conductivity meter with the option determining the content of Total Dissolved Solids (TDS).
The quality of tap water varies greatly. Especially when we are talking about water for industrial use. Figure 1 shows two tap waters taken from two paint production plants. In the beaker on the left, the water is clear and no foreign matter is visible, while in the beaker on the right, turbidity and suspended solid particles are visible.

Figure 1. Two tap waters, solids and turbidity visible on the right
Therefore, water is classified according to hardness, which takes into account dissolved cations that affect its hardness. Table 3 shows an example US water hardness classification (NSF/ANSI 44 and NSF/ANSI 330).
| Table 3. US water hardness classification | ||
| Water classification | Grains per US gallon (gpg) | mg(ppm) Ca2+/L |
| Soft | Less than 1 | Less than 17.1 |
| Slight hard | 1 – 3.5 | 17.1 – 60 |
| Moderate hard | 3.5 – 7.0 | 60 – 120 |
| Hard | 7.0 – 10.5 | 120 – 180 |
| Very hard | 10.5 and over | 180 and over |
In turn, in Europe, the water hardness classification indicated in Table 4 is often used.
| Table 4. An example of a popular European water hardness classification | ||||
| Water classification | mval/L | mg CaCO3/L | °dH | mmol/L |
| Very soft | Less than 2 | Less than 100 | Less than 5.6 | Less than 1 |
| Soft | 2 – 4 | 100 – 200 | 5.6 – 11.2 | 1 – 2 |
| Slight hard | 4 – 7 | 200 – 350 | 11.2 – 19.6 | 2 – 3.5 |
| Hard | 7 – 11 | 350 – 550 | 19.6 – 30.8 | 3.5 – 5.5 |
| Very hard | 11 and over | 550 and over | 30.8 and over | 5.5 and over |
In the technical materials attached to the raw materials and in the literature, you can find a theory about dispersion involving the building of a double electrical layer on the dispersed pigment particles, the miscibility of various coalescents with different polarities with water, or the hydration in water of phyllosilicate thickeners with negatively charged layers on the surfaces and slightly positively charged on the edges. In turn, in the technical data sheets of titanium dioxide pigments and in the ASTM D476 standard classifying the parameters of various types of titanium dioxide, the resistivity of the water extract also refers to charges and electricity.
These few examples already show that the relationships between electric charges and the efficiency of raw materials are clearly visible and must be important. Since the efficiency of raw materials depends on how electric charges will behave, it will also matter in what environment these charge exchanges will take place.
So it is in coating tests using water. If the resistance of coatings is tested and they are washed with water, various compounds migrate from their surface into the water, mainly surfactants, and as a consequence of further erosion of the coating with water, also the binder and solid particles of pigments and fillers, contaminating the water during the test. Such water no longer affects the coating with the same aggressiveness as “pure” water at the beginning.
The parameter that connects all these relationships with a common key is electrical conductivity (EC). This property is measured according to ASTM D1125 and defined by this standard as the reciprocal of the a-c resistance in ohms measured between opposite faces of a centimetre cube of an aqueous solution at a specified temperature. The unit of electrical conductivity is siemens per centimeter (S/m, for water usually expressed in µS/cm). In turn, electrical resistivity is the a-c resistance in ohms measured between opposite faces of a centimeter cube of an aqueous solution at a specified temperature. The unit of electrical resistivity is ohmcentimeter (W · cm). Electrical conductivity measurement is performed in Spektrochem lab using a Mettler Toledo SevenDirect SD23 conductivity/pH meter (Figure 2) using a sensor regularly calibrated with a conductometric SRM (Standard Reference Material) of known conductivity.

Fig. 2 Measurement of demineralized water conductivity using Mettler Toledo SevenDirect SD23
Water hardness vs conductivity
Water hardness is closely related to its conductivity. While analytical methods involving titration with appropriate reagents are used to determine water hardness as the sum of the ions responsible for hardness dissolved in it, mainly Ca2+ and Mg2+, conclusions can also be drawn about water hardness based on measurements of electrical conductivity using a conductivity metre . The electrical conductivity determined in this way according to ASTM D1125 (method A – non-flowing samples, method B – in-line samples), expressed in µS/cm, can be a quick indicator of the hardness of the water we are dealing with. Figure 3 shows the results of measuring the hardness of water with a known concentration of Ca2+ ions converted into mg CaCO3 and the hardness determined thereby in the Spektrochem lab, measuring the conductivity using a conductivity/pH meter Mettler Toledo SevenDirect SD23, as above.
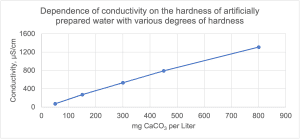
Figure 3. Conductivity vs. hardness of water prepared with a known concentration of calcium ions
For laboratory tests, which further show how to approach the hardness and conductivity of water used in the production of paints, a series of water with artificially obtained hardness was prepared to control its repeatability of use in tests. The water was prepared by preparing a stock solution with a specific Ca2+ concentration, and then graded solutions were prepared from it, from very soft water to very hard water, with hardness calculated and determined on the basis of titration, which is presented in Table 5. The waters thus prepared had different hardness according to the EN standard were stored in bottles shown in Figure 4.

Figure 4. Artificial water with graded calcium hardness
| Table 5. Artificial waters with various degrees of hardness for further testing | ||||
| Water classification | mg CaCO3/L | mmol Ca2+/L | °a | °f |
| Very soft | 50 | 0.5 | 2.925 | 5 |
| Soft | 150 | 1.5 | 8.775 | 15 |
| Medium hard | 300 | 3.0 | 17.55 | 30 |
| Hard | 450 | 4.5 | 26.325 | 45 |
| Very hard | 800 | 8.0 | 46.8 | 80 |
Water for paint production
Water for paint production should, above all, be microbiologically clean (Figure 5). This is important from the point of view of introducing microorganisms into the paint which, despite the addition of in-can preservatives, can cause deterioration caused by bacteria, fungi or yeast.

Figure 5. Quick microbiological tests to easily check the microbiological purity of water
Figure 6 shows examples of tap water collected from two production plants that contained a large number of microorganisms. Such quick microbiological tests performed by the in-plant laboratory allow us to identify the type of microorganisms in the water in order to select the appropriate preservative to eliminate them, as well as to protect against further in-can growth, as well as to monitor the purity of water in the production process, especially when reusing water after recycling, where the presence of microorganisms may be particularly common. As you can see on the bacteria plate, bacteria grow in both waters, but it can certainly be said that they are different in the type of bacteria and in intensities. In turn, yeasts/fungi plate growth occurred only in water taken from Plant A, which perfectly illustrates how important it is to perform this type of tests to avoid introducing microorganisms into paints at the stage of their production.
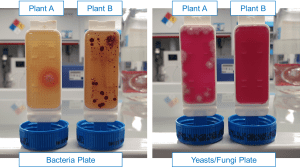
Figure 6. Growth on a quick microbiological test in water from two paint factories
The next step is to clarify what type of water in terms of hardness and conductivity should be used for paint production. You may often hear questions about whether you should use tap water or demineralized water for the production of latex paints. You can also often hear statements that using demineralised water is better, because it is purer and this may be due to the fact that in starting point formulations, demineralised water is usually found as the first item in the formulation. Both approaches are correct and wrong at the same time. How is this possible? I will discuss this with examples from the results of formulation development work.
Dispersing agents and grinding process
In order to check the influence of water hardness on the effectiveness of dispersants and the dispersion process, a mill-base consisting of titanium dioxide and calcium carbonate was prepared and ground using two doses of the dispersant in water with the addition of in-can preservative and a defoamer. Mill-base was prepared with a solids content of 80 wt%, separately in demineralized water (conductivity 1.1 µS/cm) and in very hard water 8.0 mmol Ca2+/L (conductivity 1.140 µS/cm). In order to assess the effectiveness of dispersants, mill-base viscosity measurements were made for dispersant doses of 0.25% and 0.50% (calculated on mill-base solids) and the results are presented in Figure 7.
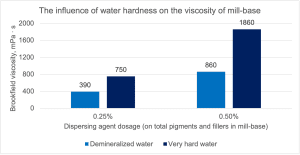
Figure 7. Comparison of dispersant efficiency in two doses in DI water and very hard water
The chart shows that the dispersant used in the tests is much more effective in demineralised water than in hard water, because the viscosity of mill-base is much lower (at a dose of 0.25%, slurry in DI water has an almost twice lower viscosity, and at a dose of 0.50% it is more than 2 times). As we know, the selection of the dose and type of dispersant is carried out in the first step of testing by finding the dosage range that ensures the lowest mill-base viscosity, because then the process of flow and grinding of particles is the most effective, and it is clearly visible that in the event of a change in water hardness, different dispersion conditions can be obtained, following which it is necessary to change the energy during dispersion and grinding time. Such changes in water hardness are particularly important when transferring formulations between production plants within one country or abroad, where water of a different hardness may be used.
We have hard water… do we need to soften it?
Another issue encountered in the paint industry is the statement “we produce hard water and we soften it by adding Calgon”. This approach can be found because there is a preparation with the same name on the market for protecting washing machines against limescale, as well as a phosphate-based dispersing agent for latex paints. Unfortunately, this leads to a very serious cognitive error and the repetition of a kind of myth that should be finally clarified.
Unfortunately, the two products on the market work completely differently and have different compositions. The one for the washing machine is a mixture of zeolites, polycarboxylates, non-ionic surfactants with a fragrance composition, and the one that is a paint dispersant is slightly alkaline sodium polyphosphate of medium chain length. Adding this dispersant in a random dose (as I usually notice) with the belief that the water should be softened is a procedure that brings nothing, apart from possible problems with dispersion or later paint stability caused by the failure to determine the dose of this agent. Polyphosphate dispersant is an excellent wetting and dispersing agent, however, when used correctly, i.e. its dose is determined by searching and finding the effective area for specific pigments and fillers. Is it really a water softener? We checked it and the results are shown in Figure 8.
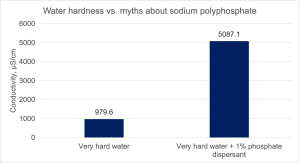
Figure 8. Does phosphate dispersant reduce water hardness?
We added 1% phosphate dispersant in powder form to very hard water (hardness approx. 7 mmol Ca2+/L, conductivity approx. 980 µS/cm, TDS 627) and mixed it with a magnetic stirrer. A sample with 1% phosphate dispersant showed a conductivity of over 5,000 µS/cm and a TDS of 3,250. As can be seen from the conductivity and TDS (Total Dissolved Solids) results, water hardness has not decreased in any way.
So, should you reduce water hardness in some other way? Should water be hard or soft? See the rest of the article and you will find the answer to this question with the help of my case studies discussing the issue cross-sectionally.
Defoamers
Defoamers are a mandatory ingredient of waterborne paints, including latex paints. They are responsible for reducing foam during the production process, pouring, storage, application, etc. More and more often we are dealing with new “green” defoamers, and therefore new additives for which application research is still being conducted. I have prepared a technical article about this in more detail here. As we know, surfactants stabilise foam in water. These surfactants are dispersants in paint, surfactants from polymer dispersions and other additives contained in the formulation, and even being a component of surface treatment, e.g. titanium dioxide. But do defoamers always work the same? Will their effectiveness depend on water hardness and conductivity?
Soft water has much lower surface tension than hard water. This means that surfactants dissolve well in it and if they have foaming properties (and not every e.g. dispersant has foaming properties), it means that the foaming and stability of this foam will be higher in water of lower hardness.
Figure 9 shows the foaming of very soft and very hard water in graduated cylinders with the addition of 0.5% of a dispersant with strong foaming properties. As you can see, the difference in foam stability is clearly visible. In the cylinder on the left, in very soft water, the foam has a larger volume and remains stable, but in the cylinder with very hard water on the right, you can see that the foam loses stability and its volume decreases. This means that surfactants foam more in water with lower conductivity, in soft water. But can the same be said about paints? Can the action of defoamers be directly linked to this?

Figure 9. Foaming properties of the dispersant in very soft and very hard water
In order to determine the influence of the hardness of water used to produce latex paints, two latex paints were prepared to which the same defoamer was added, and each paint was prepared separately using demineralised water and tap water. A foaming test was performed for them using a foam roller, and the results of foam stability on a wet coating are shown in Figure 10.
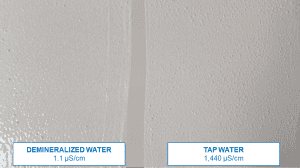
Figure 10. Foaming properties – roller test for paints prepared with DI water and hard water
As you can see, the paint sample with demineralised water shows much fewer bubbles compared to the paint coating prepared with very hard tap water. This means that we have a completely opposite situation than that presented in the cylinders in Figure 9, where greater foam stability was achieved for demineralised water. This is because there are other ingredients in the paint formulation that significantly affect the effectiveness of defoaming, especially for specific types of defoamers. A case like the one in Figure 10 shows that if hard water is used for paint production, it would be necessary to consider replacing the defoamer with another one and repeating the effectiveness tests, or introducing an additional defoamer added at the let-down stage, which would destroy the foam resulting from the high hardness of the water.
The effectiveness of defoamers depends strictly on the method of their introduction, especially when dealing with defoamers based on mineral oil or silicone (duration of shear forces, speed during introduction, dosing stage, etc.). You can read more about this in my other technical articles on the correct use of defoamers. However, if their incorporation into the system is done correctly and is based on effectiveness tests, susceptibility to the influence of water hardness may be noticeable anyway, depending on how much the foam is stabilised by the surfactants present in the paint. Figure 11 shows a comparison of three defoamers in a stirring test in which samples are aerated at high stirring speeds and then their weight per gallon is compared to samples not subjected to foaming.

Figure 11. Comparison of changes in weight per gallon of paint samples with DI and hard water after foaming tests at high speed mixing
As you can see, the paints before foaming have a weight per gallon of 10.1 lbs/US gal. After the stirring test, the most effective defoamer was No. 1, in which the difference between the sample with DI water and hard water is the smallest, however, a slight advantage of DI water over hard water is visible. In the case of defoamer No. 2, the defoaming efficiency is much weaker, and in addition, despite the poor result in general, it can be noticed that the paint with DI water shows stronger foaming than the same paint with hard water. In the case of defoamer No. 3 it can be said that the differences are very small between DI water and hard water. This example chart shows how much the effectiveness of defoamers themselves can vary, each of them has a different chemical base, but also within each defoamer you can notice some differences in effectiveness resulting from the hardness of the water used for paint preparation. Being aware of this and taking into account the influence of water on foaming properties is an important point when considering the selection of defoamers.
Phyllosilicate thickeners
Phyllosilicate thickeners are a very broad group of rheological additives for latex paints, particularly attractive for application in medium and low PVC, including bentonites, attapulgites, smectites and other swelling layered silicates. Their use in formulations is not the easiest due to the need to properly prepare hydrated suspensions and properly correlate other rheological additives with them, however, they provide extremely valuable rheological properties, especially in the area of low- and mid-shear forces. You can read my technical article about phyllosilicate thickeners using the example of smectites here.
For phyllosilicate thickeners to fulfill their role, their silicate layers must be separated using the hydration process that occurs in water under the influence of shear forces. The change in electric charge on the surfaces of these plates and their edges is very important, therefore the influence of conductivity and, consequently, water hardness is not indifferent. It is not enough to apply high shear forces to create an effective viscosity-building gel, you also need to know which phyllosilicate thickener requires water with lower and which with higher conductivity to ensure appropriate rheological performance in the paint.
In the examples below, I have discussed part of the knowledge in this area, necessary to understand how important the formulator’s experience in working with these thickeners is. Let’s start by preparing pre-gels from attapulgite and bentonite. Let me emphasize here that the complexity of working with these thickeners is so great that it does not mean that every attapulgite or bentonite will behave the same, in fact, I can say with great certainty that each bentonite or attapulgite, depending on the deposit or processing, will show different behaviour.
Figure 12 shows the viscosity measurement results of 10% attapulgite pre-gels prepared in DI water and very high hardness water. As you can see, the differences in viscosity both immediately after hydration and after 48 hours of preparation are huge. The attapulgite used in the research does not form any gel in demineralized water and the viscosity of the suspension is very low. In hard water, which contains calcium or magnesium ions, the same attapulgite has a viscosity that is over 25 times higher than the sample prepared in demineralised water.
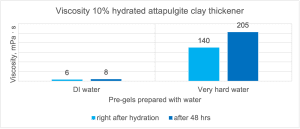
Figure 12. Comparison of viscosity of attapulgite pre-gels in DI water and hard water
Figure 13 shows a similar situation but with prepared bentonite pre-gels in the form of a 4% suspension. Samples in demineralised water show lower viscosity, but not as low as the previously discussed attapulgite, which means that the tested bentonite shows swelling properties in demineralised water. However, in this case, a much higher level of hydration was also achieved for hard water, which can be seen in the graph and is caused by the presence of ions that facilitate the separation of the silicate layers.
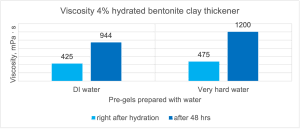
Figure 13. Comparison of viscosity of bentonite pre-gels in DI water and hard water
After the above tests of pre-gels, you already know that generally phyllosilicate thickeners show better hydration when the water in which it takes place is harder, due to better conductivity and exchange of electric charges. But do pre-gels prepared in this way have the same impact on the viscosity of paints? We checked this in the formulation of the 34% PVC latex paint, preparing the paints separately in DI water and hard water (the same as for the preparation of pre-gels) and then respectively equalising each of them in the let-down process of attapulgite and bentonite pre-gels, in DI water and hard water. The results are presented in Figure 14 for paints with attapulgite and Figure 15 for paints with bentonite.

Figure 14. Comparison of Brookfield viscosity of paints with attapulgite thickener based on DI water and hard water
As can be seen in Figure 14, the viscosity of the paint prepared with attapulgite is higher in demineralised water than in hard water. We are dealing here with a completely different situation than in comparison to the obtained pre-gels, for which the viscosities were completely opposite to those of the obtained paint viscosities. These results show that despite the same level of attapulgite dosing in the formulation, the obtained viscosities are different and completely different from the viscosities obtained for the prepared pre-gels. This happens because the remaining paint ingredients cause fluctuations in the viscosity of the final paint. When we analyse the viscosity of paints with bentonite, in Figure 15 we will see that the paint with bentonite in hard water has a significantly higher viscosity compared to the paint with demineralized water, which in the case of the tested bentonite sample is consistent with the results obtained for the viscosity of pre-gels in DI water and hard water.

Figure 15. Comparison of Brookfield viscosity of paints with bentonite thickener based on DI water and hard water
The presented results on the influence of the viscosity of phyllosilicate thickeners in the form of suspensions prepared for addition to latex paints, as well as the viscosity of the paints themselves, are not so obvious for interpretation and depend strictly on the type of silicate thickener, conductivity and water hardness values, as well as the formulation. This analysis shows that the approach to the issue requires extensive knowledge and an expert approach that requires relying on extensive case studies to obtain the most efficiency from thickeners of this type.
Polymer dispersions and stability
Polymer latex binders obtained by emulsion polymerization of monomers in an aqueous medium are formed into particles using surfactants and are also stabilized against uncontrolled coagulation. These surfactants, depending on whether they are anionic, cationic or non-ionic, show varying stability to various mechanical and chemical factors (Figure 16). One of the destabilisers of surfactants are Ca2+ ions, which are used in the form of solutions with various concentrations of these ions to test the chemical stability of surfactants after use in the polymerization process and preparation of polymer dispersions.
As mentioned many times above, Ca2+ ions, in turn, are responsible for the hardness of water and it is not difficult to conclude that in terms of surfactant stability, polymer dispersions are produced in demineralized water, this water will be more friendly to polymer dispersions in terms of surfactant stability when we produce latex paints based on it.
Destabilization of surfactants in polymer dispersions, depending on the type of surfactants and polymer dispersions, occurs at relatively high levels of Ca2+ ion concentration, e.g. about 3 mol/L, although there are polymer dispersions on the market that coagulate at a concentration of 0.1 mol/L, and even which are completely stable at 3 or 5 mol/L. As a reminder, in water with very high hardness, the concentration of Ca2+ ions may be, for example, 8-10, but mmol/L (millimoles). So, are polymer dispersions safe in terms of coagulation and stability even in hard water? Not completely.

Figure 16. Coagulation of polymer latex as a result of destabilisation of surfactants with Ca2+ ions
Destabilisation in the form of strong coagulation of the polymer dispersion may, of course, occur in extreme cases in very hard water and when using a polymer dispersion with low chemical stability for Ca2+ ions, however, from the point of view of most cases, another type of destabilisation is dangerous here and must be taken into account when designing paint formulations using specific raw materials.
In hard water, where the concentration of Ca2+ ions is high, the coagulation process may be so mild that it will not result in the formation of cottage cheese, but in the form of viscosity drift and increase in viscosity over time, syneresis, or a problem in forming a coating with the desired scrub resistance. Figure 17 shows a graph of viscosity changes for two polymer dispersions with different stability of their surfactants to Ca2+ ions, which were used as binders in latex paints and tested for viscosity stability over time.
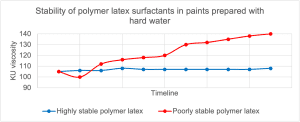
Figure 17. Comparison of viscosity stability of paints based on two polymer dispersions in terms of the influence of surfactants on stability
As you can see, the polymer dispersion in the blue graph, showing high stability of surfactants to a high concentration of Ca2+ ions, used in paint with hard water (water hardness 12 gpg) shows very good viscosity stability in the mid-shear range (KU viscosity) throughout the entire storage stability test period. In turn, the paint shown in the red diagram, which uses a dispersion of polymers with low stability of surfactants for Ca2+ ions, shows an increase in viscosity up to the limit of determination of KU viscosity.
Water for coating tests
Water is also used to test paint coatings after they have dried. Both for preparing various solutions in chemical resistance tests, e.g. for acids or bases, but above all for testing resistance to water itself. These tests are usually performed to specific standards, but it is not always clear what type of water should be used for such tests. Standard tests in which water is one of the factors causing washing out, thermal shock or swelling of coatings are accelerated weathering tests in a UV chamber or xenon-arc chamber, as well as the resistance of coatings to immersion in water.
Accelerated weathering tests
Accelerated weathering tests are performed to check the tendency of coatings to lose their properties as a result of intensified artificial radiation (UV or xenon-arc filtered light), and to accelerate changes in the form of blistering, leaching or shock lowering of sample temperature, additional cycles with water are also carried out in the form of condensation or water-spray. The water used for such tests is usually defined as demineralised, but as you already know from the previous part of the article, there is no such thing as one type of demineralized water. The ASTM standards for exposure in the UV chamber (Figure 18) and xenon-arc chambers define the requirements for the water that should be used for testing, as well as when to replace this water to maintain its properties. It is not surprising that demineralized water, while losing its conductivity and pH, also loses its aggressive washing effect, which is why it is so important to monitor the conductivity and pH of water during accelerated weathering tests and replace water with water-spray in accordance with ASTM requirements, which are defined as conductivity below 5 µS/cm, contains less than 1-ppm solids and silica content below 0.2 ppm. Additionally, water pH monitoring is required during the test and reported.

Figure 18. Water-spray nozzles in the QUV chamber in the Spektrochem lab
This means that it is not possible to use water with a higher conductivity than that indicated in the ASTM guidelines, which is especially important when using recirculation in a UV or xenon-arc chamber. Water must be replaced as often as the conductivity changes above 5 µS/cm, and it usually changes very often in the initial phase of the water-spray test, and when we are dealing with samples that tend to leach substances intensively. Figure 19 shows the water tank from the QUV chamber during the first water-spray cycle, showing foam on the water surface caused by various components being washed out of the coatings during the test. Such water has a conductivity above 19 µS/cm and must be replaced before the next water cycle and monitored every subsequent one. Therefore, a conductivity meter and a pH meter are mandatory equipment in a weathering laboratory.

Figure 19. Water in the recirculation tank in the QUV chamber after the first water-spray cycle
Monitoring water quality during weathering tests is crucial both in terms of pH and conductivity, basically only in the case of water-spray. During condensation, the process of water evaporation causes the coatings to be affected by water without the ingredients dissolved in it, but remember to always use water of known quality. Otherwise, the results obtained may be very unreliable, especially since the water used in the weathering chamber is responsible for many coating changes, e.g. blistering (Figure 20).

Figure 20. Blistering on coatings during exposure in the QUV chamber caused by the impact of demineralised water
Table 6 shows sample data from QUV chamber testing at the Spektrochem lab during an ASTM water spray exposure cycle, showing how frequently water quality monitoring and water changes were performed to maintain ASTM standards. Water quality measurements in the Spektrochem lab shown in Table 6 were made using ASTM standards and a SevenDirect SD23 by Mettler Toledo pH meter/conductivity meter (Figure 21).
| Table 6. Examples of water quality monitoring after exposure cycles in a QUV chamber | |||||||
| Parameter | A) | B) | C) | ||||
| pH | 6.23 | 6.06 | 6.33 | 6.10 | 6.24 | 5.87 | 5.76 |
| Conductivity, µS/cm | 19.50 | 13.72 | 14.22 | 1.21 | 1.48 | 3.46 | 2.44 |
| Resistivity, kW · cm | 51.33 | 72.88 | 70.30 | 891.7 | 637.2 | 289.2 | 409.0 |
| TDS, mg/L | 9.74 | 6.86 | 7.11 | 0.56 | 0.74 | 1.73 | 1.22 |
| TDS, % | 0.007 | 0.008 | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 |
| Changing the water in the tank after cycle | Yes | Yes | Yes | No | No | No | No |
| Initial water parameters: pH 6.00, conductivity 1.5 µS/cm
A) Measurements of water parameters after the first spray cycle B) Measurement of water parameters after replacement after the next cycle C) Measurements on subsequent days of exposure |
|||||||

Figure 21. Measurement of the pH of water exposed to a QUV chamber using a pH meter and a combined electrode
Water immersion tests
Another test in which water quality is crucial is the water immersion test, which is usually carried out to determine the resistance of coatings to matting (loss of gloss as a function of immersion time in water) or resistance to blistering. The ASTM test is carried out in water at a temperature of 100 °F for a time depending on the required resistance. The ASTM test method, which provides guidelines for conducting this test, requires water quality control for conductivity and discharge when it is higher than 20 µS/cm (resistivity less than 50 kW · cm). This is important from the point of view of the aggressiveness of the test and the migration of components leachable from the coating into water.
In the case of R&D work, measuring the water resistance of coatings, e.g., wood or metal, by testing them immersed in water at a temperature of 100 °F is standard, and therefore maintaining water quality throughout the entire test is very important. Therefore, monitoring of water conductivity in our laboratory is carried out every 24 hours if the water resistance lasts several days or longer, and every 2 hours if the test lasts from e.g. 4 hours to 10 hours. In Figure 22 and 23 you can see the reading results respectively conductivity of demineralized water before and after tests of resistance to immersion of coatings in water after 6 hours. If coatings with good water resistance are tested, where there is no migration of components from the coating, then the water conductivity result remains low even after the tests are completed. However, in R&D and QC work we do not always deal with high-quality coatings and water quality monitoring is very important.
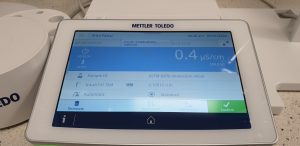
Figure 22. Water conductivity measurement before water immersion test

Figure 23. Water conductivity measurement after water immersion test
As another example of the results of immersion coating tests, Figure 24 shows the effect of the importance of controlling water conductivity and its replacement in water immersion tests on changes in the gloss of wood coatings.

Figure 24. The importance of monitoring and water replacement during water immersion tests and its impact on gloss
The chart includes gloss measurements for wood coatings marked as Wood coatings #1 and Wood coatings #2. The initial gloss of the coatings is changed by immersion for 24 hours in water at 100°F with water aeration as per the ASTM standard. As you can see in the graph, the test was performed without changing the water and during the test the water reached a conductivity of 35 µS/cm (middle columns of the graph), as well as with changing the water during the test and maintaining the conductivity at 12 µS/cm (columns on the right side of the graph ).
There is a drastic difference in the decrease in the gloss of the coatings, which decreased not so significantly when the water was not changed during the test, despite achieving a conductivity above the limit of 20 µS/cm, compared to the gloss after the end of the 24-hour immersion test when the water was controlled and changed to maintain required level of conductivity. If the test was performed correctly, the gloss of the Wood coating #1 coating decreased by more than 7 times, and if the test was performed incorrectly, it decreased by less than 2 times. These results show that performing tests taking into account water quality monitoring and correctness of passing ASTM tests is crucial for the appropriate aggressiveness and reliability of the test.
Summary
I hope that everyone has reached the summary, despite the length of the article, which discussed the issue of water in waterborne paints cross-sectionally and quite generally, both in terms of production and testing of their resistance. So what is the answer? Demineralized or tap water, hard or soft? It depends. It depends on how specific raw materials used in the paint to formulate the recipe will tolerate a particular type of water in terms of stability, in which type of water better dispersion efficiency, viscosity building, ingredient precipitation and finally foam destruction will be achieved. Such information on what type of water a given raw material is more effective in is the work of the Spektrochem R&D laboratory, where every day we prepare documentation of raw materials to find their best performance in waterborne paint formulations. In turn, for testing paints, and more specifically their coatings, demineralized water must be of appropriate quality, monitored during the test and changed in accordance with the guidelines of quality standards, so that the tests are repeatable and reliable.







