More news
- Focus on the global coatings market: Global coatings market outlook
- View from the UK: Navigating chemical policy and sustainability
- Focus on adhesives: Unveiling unbreakable bonds – Testing redefines physical strengt...
- Focus on adhesives: Henkel and Covestro collaborate for sustainability of engineered wood ...
- Advances in construction chemical technology: What’s new in 2024?

Piet Driest, Richard Brinkhuis, Daan Willink, Tonny Buser, Robertino Chinellato and Florian Lunzer, Allnex Global R&D, introduce a novel high-speed crosslinking technology for powder coatings based on Michael addition chemistry
The popularity of powder coatings can be explained by four ‘E factors’: Ecology, Ease of application, Economy of use and Excellence of finish. [1, 2] However, conventional powder coatings require a relatively high curing temperature of 160-200°C. Not only is this unfavourable from a sustainability point of view, but it also prohibits the application of powder coatings to heat-sensitive substrates such as MDF, wood or plastics. Therefore, the development of powder coatings that can be cured at lower temperatures has gained much attention over the past years. Specifically, curing temperatures below 130°C are of interest, since below this threshold application to MDF and engineering plastics becomes feasible. Typically, this lower temperature cure (LTC) regime (also known as ultra-low bake) cannot be achieved by conventional crosslinking technologies and requires alternative, more reactive, chemistry. Since typical processing (e.g. extrusion) temperatures are not far below the target cure temperature, the use of reactive chemistry is technologically challenging. Also, at lower temperatures the melt viscosity of the paint is much higher, whereby viscosity quickly increases further once the crosslinking process starts. As a result, the total paint flow is typically very limited for highly-reactive LTC powder systems, which negatively impacts e.g. wetting of the substrate, film formation and appearance.
Various reactive crosslinking chemistries have been used in the past to design LTC powder systems (e.g. epoxy-acid/anhydride, blocked isocyanate, radical polymerisation), all of which are limited by the trade-off between reactivity and paint flow. A special case worth mentioning in this context is the use of UV-curable powder systems, for which the flow and crosslinking stages are decoupled. However, not all applications are suitable for a UV-curable setup.
In the past, we have successfully introduced Michael addition chemistry as a fast crosslinking reaction for ambient-cure solventborne and waterborne systems. [3, 4] Building on this experience, we hereby introduce the use of Michael addition chemistry as a novel highly-reactive crosslinking technology for LTC powder paints.
READ MORE:
Reintroducing the wheel: Linseed paint as the natural option
Real Michael addition as a highly reactive crosslinking reaction for powder coatings
Real (or carbon) Michael Addition (RMA) chemistry features a nucleophilic addition reaction between an acidic C-H (the Michael donor) and an electron deficient C=C unsaturated bond (the Michael acceptor), creating a new C-C bond (see Figure 1). The process is catalysed by a strong base, able to deprotonate the Michael donor. Once catalysed, the RMA reaction can proceed very rapidly (even at room temperature), whereas without an active catalyst there is essentially no reactivity between the two reaction partners. This strong contrast makes the Michael addition reaction a very suitable candidate for a highly reactive crosslinking reaction for powder coatings. In terms of molecular design, typical functional groups that can act as a Michael donor include e.g. malonates, acetoacetates and cyanoacetates. Typical functional groups that can act as a Michael acceptor include e.g. acrylates, methacrylates, maleates, fumarates and itaconates. Binders for powder paints containing these functional groups can be designed with various equivalent weights, functionalities and different types of backbones (e.g. polyester, urethane, epoxy, acrylic), spanning a wide range of material properties.
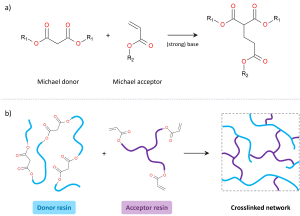
Figure 1. The use of (real) Michael addition as a highly reactive crosslinking reaction for powder coatings; a) general Michael addition reaction between a Michael donor and a Michael acceptor (example shown here for a malonate donor and an acrylate acceptor). b) Crosslinking based on Michael addition chemistry, combining a Michael donor-functional resin with a Michael acceptor-functional resin. In absence of a strong base no reaction takes place, whereas in presence of a strong base very fast crosslinking is achieved
The catalyst package: introduction of a pre-catalytic cycle and the advantages of delayed cure
One of the essential components of the new Michael addition-based crosslinking technology is a multi-component catalyst package, which is designed to enable control over the reactivity in time. This multi-component system consists of a catalyst precursor, an activator and a retarder (see Figure 2).
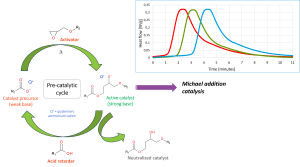
Figure 2. A multi-component catalyst package is used for a delayed onset of the Michael addition reaction. Inset: differential scanning calorimetry (DSC) thermograms of three identical powder paints containing increasing amounts of acid retarder (isothermal, 120°)
The catalyst precursor consists of a quaternary ammonium carboxylate salt, which is not basic enough by itself to trigger the RMA reaction. At curing temperature, this carboxylate can react with an activator component (typically an epoxide) to form a strongly basic alkoxide adduct. This adduct is basic enough to deprotonate the acidic C-H moieties of the Michael donor and can thereby initiate crosslinking. However, if a carboxylic acid (retarder) is also added to the system, this carboxylic acid will be deprotonated preferentially before the Michael donor, re-forming a carboxylate salt which can then re-enter the pre-catalytic cycle. As such, the onset of the Michael addition reaction is delayed until all the acid retarder present in the system has been consumed first. In this way, a delayed curing profile is created, whereby the amount of delay time (i.e., induction time) can be controlled by varying the amount of acid retarder added to the system (see Figure 2, inset). The benefit of such a delayed cure profile is two-fold: a) the risk of premature crosslinking in the extruder during processing of the powder paint is minimised; and b) during the induction period, a window of high fluidity is created, resulting in a high total paint flow. Since the total amount of paint flow is directly related to PCI [5], this offers a great tool to control the PCI independently of curing speed. This can be seen as analogous to the decoupling of flow and cure stages offered by UV-curable powder coatings, but without the need for additional UV infrastructure.
READ MORE:
Beckers Group appoints Nicklas Augustsson Chief Sustainability Officer
Coating performance
To demonstrate the performance of RMA-based powder coatings, two representative RMA-based powder formulations were prepared in white and applied on MDF (characteristics summarised in Table 1). A typical curing profile of 6 minutes at 130°C was selected, using infrared (IR) heating. The same donor resin was used in both paints, which was a malonate polyester with an equivalent weight (EQW) of 1000 g/mol. As for the acceptor, paint 1 was based on an epoxy-acrylate (acrylate EQW of 600 g/mol), whereas paint 2 was based on a urethane-acrylate (acrylate EQW of 400 g/mol). A single layer of powder coating was applied directly onto MDF and cured. Based on DMTA and DSC measurements, it was seen that the resulting coatings both demonstrated a high crosslink density and glass transition temperatures (Tg). In line with this, excellent solvent-, stain- and scratch resistance were observed. Next, the outdoor durability of a brown equivalent of paint 2 (RAL8014, 30% pigment) was measured by accelerated Q-UVB test (in this case, an outdoor durable epoxy was used as the activator). It was observed that >400 hours of Q-UVB were achieved before losing 50% in gloss.
Table 1. Coating characteristics of two RMA powder paints applied on MDF:
| Paint 1 | Paint 2 | |
| Curing condition: 6 minutes @130°C (IR heating) | ||
| Paint Tg [°C] | 46 | 51 |
| Layer thickness [µm] | 80-100 | 80-100 |
| Gloss [60°] | 90-97 | 90-97 |
| Solvent resistance (DIN 6886-1) | ||
| Acetone (10s) | 4 | 4 |
| MEK (50 rubs) | pass | pass |
| Stain resistance (EN 12720) | ||
| Water (24h) | 5 | 5 |
| Fat (24h) | 5 | 5 |
| Coffee (6h) | 5 | 5 |
| 48 % Ethanol (6h) | 5 | 5 |
| Adhesion (EN ISO 2409) | Class 1 | Class 1 |
| Wet heat resistance (EN 12721) | 5 | 5 |
| Dry heat resistance (EN12722) | 5 | 5 |
| Scratch resistance, 5N (SS 839117) | 4 | 4 |
| Scratch and fat resistance, 5N/24h (SS 839122) | 4 | 4 |
| PCI smoothness | 4-5 | 2-3 |
| DMTA crosslinking density [mmol/cc] | 0.53 | 0.44 |
| DSC cured film Tg [°C] | 86 | 95 |
Conclusions
We have developed a novel toolbox for a highly reactive crosslinking technology for (indoor and outdoor) powder coatings based on Michael addition chemistry. An integral part of this new technology consists of a multi-component catalyst system, which offers control over the reactivity of the system in time. As a result, fast low temperature cure (down to 100°C) can be combined with good workability and improved appearance. This allows for effective application to heat-sensitive substrates such as MDF or plastics. Alternatively, application on conventional substrates at higher temperatures can be performed in shorter times. The resulting coatings show good mechanical and thermal properties (e.g. solvent-, scratch- and stain resistance, crosslinking density, Tg and weatherability).
Acknowledgements
The authors would like to express their gratitude to everyone who has contributed to this work: A. Campana, M. Censi, P.J. Elfrink, M. Zancope Ogniben, M. Turrin.
References
[1] Bocchi G J., Modern Paints and Coatings, 76 (1986), 44
[2] DSM powder coating resins, DSM Carbon Footprint Study for Industrial Coatings Applied on a Metal Substrate
[3] Brinkhuis R. et al, European Coatings Journal, 5 (2015), 34-40
[4] Temel A., Lunzer F., European Coatings Journal, 11 (2019), 24
[5] Yang P. et al., European Coatings Journal 12 (2023), 18-23